Will Nitromethane Make a Comeback in Rocket Fuel?
Release time:2025-04-07
Introduction
In the field of aerospace, the selection of rocket propellants has always been a key area of research for scientists and engineers. With increasing environmental awareness and the pursuit of high-performance propellants, a once-overlooked compound—Nitromethane (NM)—is reentering the spotlight. This article will delve into the potential of nitromethane as a green rocket fuel and the latest research developments.

History and Current Status
Since the 1930s, nitromethane has been considered as a rocket fuel. It is a liquid compound at room temperature with low toxicity and good storage and transport properties. During World War II, the U.S. military conducted extensive research on its potential as a rocket propellant. Similarly, Israel explored its use in the 1950s, particularly in combination with other chemical substances to enhance combustion efficiency. However, with the rise of highly efficient yet toxic hydrazine-based fuels, research on nitromethane stagnated.
Currently, chemical satellite engines still predominantly use hydrazine, monomethylhydrazine (MMH), and unsymmetrical dimethylhydrazine (UDMH) as rocket fuels. These substances are highly toxic but offer long-term storage capabilities. Due to the stringent handling requirements of hydrazine, it is not a viable option for startups and micro-launchers in the "New Space" sector. As awareness of the health and environmental impacts of hydrazine-based fuels grows, the search for greener alternatives has intensified, bringing nitromethane back into focus.
What Are the Advantages?
- Environmentally Friendly: Compared to traditional hydrazine-based fuels, nitromethane has a smaller environmental impact and is considered a green propellant.
- High Performance: Nitromethane offers a high specific impulse, providing good propulsion efficiency.
- Ease of Storage: As a liquid fuel, nitromethane is stable at room temperature and can be stored for extended periods.
- Cost-Effective: Nitromethane is widely used as a solvent, making its cost relatively low.
What Are the Challenges?
Despite its many advantages, nitromethane faces several challenges in practical applications:
- Combustion Efficiency: While combustion efficiency in some tests has reached over 90%, it has not yet achieved 100%.
- Ignition and Stable Combustion: Nitromethane struggles with stable combustion at low pressures and requires additives or catalysts to facilitate ignition and maintain stability.
- Sensitivity: As an energetic material, nitromethane is sensitive to external stimuli and may decompose or explode upon impact or heating, necessitating desensitizers and additional safety measures.
- Propulsion System Design: The physical and chemical properties of nitromethane differ from conventional propellants, potentially requiring redesigns of existing rocket propulsion systems.
- Technology Maturity: Compared to other widely used propellants, nitromethane as a rocket fuel is still in relatively early stages of application, requiring further research and testing to validate its long-term performance and reliability.
- Safety and Regulation: Although nitromethane is a green propellant, its energetic nature means it remains subject to regulatory scrutiny.
Research Progress
Return to the Spotlight
A 2006 conference paper was one of the key factors that reignited interest in nitromethane:
Boyer E, Kuo K. Characteristics of nitromethane for propulsion applications[C]//44th AIAA Aerospace Sciences Meeting and Exhibit. 2006: 361.
This paper presented several significant research findings and future directions for nitromethane:
Based on the physical properties and propulsion performance of nitromethane, adding specific stabilizers could make it an attractive, storable, low-toxicity monopropellant.
Nitromethane can also serve as a base material for gelled propellants with the addition of other high-energy substances, such as nano-metal powders or crystalline oxidizers.
The study examined the combustion behavior of nitromethane at pressures below 15 MPa and found it highly suitable for rocket applications. Existing models effectively predict its burning rate and flame structure within this pressure range. Future model extensions to higher pressures and mixtures could provide valuable tools for propulsion system design.
In monopropellant applications, combustion instability observed in earlier studies can be mitigated using modern design techniques and materials.
Ongoing Exploration
In the following decade, interest in nitromethane as a rocket fuel continued to grow, attracting significant attention from German scientists. A 2012 German study proposed a gelled nitromethane propellant infused with metal particles and hydrides:

Weiser V, Hürttlen J, Kelzenberg S, et al. Combustion Behaviour of Gelled Nitromethane Propellants Filled with Metallic Particles and Hydrides[C]//Proc. of 15th Seminar New Trends Res. Energetic Mat., Pardubice, Czech Republic. 2012: 330-345.
Key Findings: Gelled propellants combine the advantages of solid and liquid propellants, such as leak resistance and pumpability. Nitromethane (NM), as a green colloidal monopropellant, can enhance performance by adding metal particles or other additives. The study explored nano-metal ceramic gelled propellants containing Al, B, Mg, Si, Ti, Zr, aluminum hydroxide, and magnesium hydride. While substituting aluminum and magnesium hydrides did not increase specific impulse, it improved combustion performance and reduced combustion temperature.
Recent International Studies
With the publication of a 2021 review paper on green monopropellants, research on nitromethane in rocket fuel applications entered a period of rapid development.
Nosseir A E S, Cervone A, Pasini A. Review of state-of-the-art green monopropellants: For propulsion systems analysts and designers[J]. Aerospace, 2021, 8(1): 20.
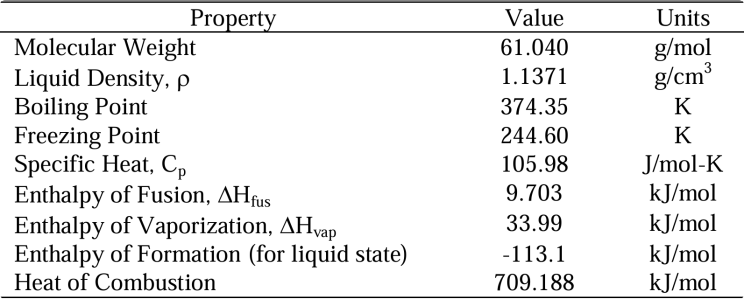
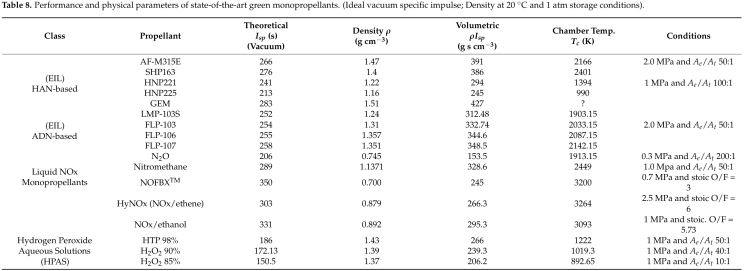
Key Content: Nitromethane (NM) is a promising green monopropellant suitable for space propulsion systems with varying thrust levels. It is a low-toxicity, viscous, liquefiable liquid with a density of 1.1371 g/cm³ and a freezing point of -28.4°C, making it easy to store in space. The addition of stabilizers enhances its appeal. NM is viable for both monopropellant and bipropellant systems, offering high performance, high volumetric specific impulse, and a combustion temperature of approximately 2449K.
In the following years, the German Aerospace Center (DLR) conducted extensive research on nitromethane-based rocket propellants, testing its first-generation formulation (NMP-001) and presenting related findings in multiple papers and conferences.
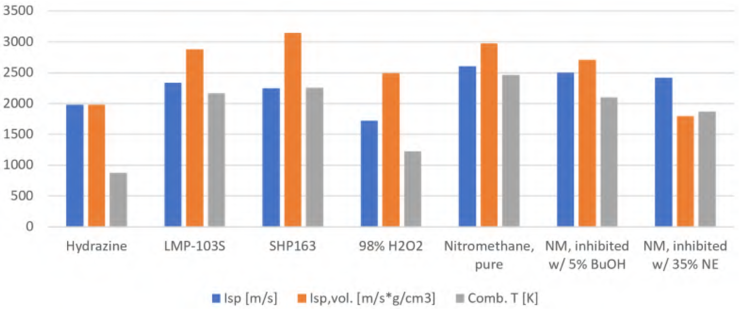
Schwind R A, Sinrud J B, Fuller C C, et al. Comparison of flame inception behavior of liquid nitromethane in inert and air environments[J]. Combustion and Flame, 2022, 241: 112101.
Key Content: As a green monopropellant, nitromethane (NM) has been insufficiently studied in oxygen-free combustion kinetics, especially in underwater and space propulsion applications. Existing research primarily focuses on oxidative environments, providing gas-phase kinetic data, but little is known about ignition and performance in inert environments. This paper presents experimental data from a novel continuous-flow liquid propellant burner designed to measure NM ignition behavior.
Werling L, Freudenmann D, Ricker S C, et al. Research and Test Activities on Advanced Rocket Propellants at DLR’s Institute of Space Propulsion in Lampoldshausen[J]. 2022.
Key Content: Nitromethane (NM) is a green propellant with excellent rocket performance, surpassing other green monopropellants. However, it is also a potential liquid explosive. To reduce the risk of explosions in the injector channels of rocket thrusters, sensitivity can be suppressed by adding gelling agents such as benzene and xylene. Although this modification slightly decreases performance, the improved NM mixture still outperforms other green propellants, offering a higher specific impulse than LMP-103S and SHP-163.
Kurilov M, Werling L, Kirchberger C, et al. Nitromethane as a Green Propellant: First Results of a Combustion Test Campaign[J]. 2023. Kurilov M, Werling L, Kirchberger C, et al. Combustion behavior of NMP-001, a nitromethane-based green rocket propellant[J]. Acta Astronautica, 2024, 223: 1-14.
Key Content: By incorporating specific additives, the nitromethane-based monopropellant NMP-001 improved low-pressure combustion performance and mechanical sensitivity. Experiments demonstrated stable combustion at 15 bar, proving that NMP-001 can generate thrust at pressures typical for satellite and spacecraft propulsion systems. Future research will focus on optimizing combustion chamber design and identifying more suitable ignition methods.
Kurilov M, Werling L, Negri M, et al. Impact Sensitivity of Nitromethane-based Green-propellant Precursor Mixtures[J]. International Journal of Energetic Materials and Chemical Propulsion, 2023, 22(2).
Key Content: The impact sensitivity and theoretical rocket performance of nitromethane-based propellants were tested using the BAM Fallhammer impact tester and NASA CEA program, respectively. The study found that adding 6 wt.% dimethyl sulfoxide (DMSO) significantly reduces impact sensitivity while only slightly decreasing the theoretical specific impulse
Kurilov M, Ziemer L, Weiser V, et al. Ignition of Nitromethane-Based Propellant Mixtures[J]. International Journal of Energetic Materials and Chemical Propulsion, 2024, 23(1).
Key Content: Although nitromethane is difficult to ignite, its combustion performance can be improved by adding additives such as acetylacetonates. Experiments show that ferrocene is particularly effective, significantly lowering the decomposition temperature of nitromethane and increasing the combustion rate, making it an ideal ignition and combustion catalyst in nitromethane-based propellants.
Ciezki H K, Naumann K W, Hürttlen J, et al. Short Overview and Highlights of the History of the German Gel Propulsion Research and Technology Development Activities[J]. International Journal of Energetic Materials and Chemical Propulsion, 2024, 23(4).
Key Content of the Article: This article provides a brief overview of the work and achievements in the German rocket propulsion field over the past 25 years, with a particular focus on the progress made in recent years.
These articles have not only had a significant impact on academia but have also prompted the German Aerospace Center (DLR) to take proactive measures. DLR has filed a series of internationally influential invention patents to ensure that its research findings are protected in terms of intellectual property. At the same time, these efforts lay the groundwork for future commercial collaborations and technology licensing.


Meanwhile, Japan has also been exploring nitromethane’s potential as a rocket fuel:
Minato R, Tanaka S. Fluid hammer phenomena for Nitromethane propellant feed system[J]. Science and Technology of Energetic Materials, 2022, 83(4): 111-116.
Key Content: At tank pressures of 0.4, 0.6, 0.8, and 0.95 MPaG, nitromethane did not explode. The pressure increase of NM closely matched theoretical predictions from the Joukowsky equation. The peak pressure of NM’s water hammer effect was equal to or higher than that of water. Since nitromethane exhibits hydrodynamic behavior similar to water, it is recommended to use water for testing fluid hammer effects in NM feed systems.
Recent Chinese Studies
In China, research on nitromethane as a rocket fuel is rapidly advancing, with multiple institutions and universities actively contributing, including Nanjing University of Aeronautics and Astronautics, Xi’an Modern Chemistry Research Institute, Southwest Jiaotong University, and North University of China.
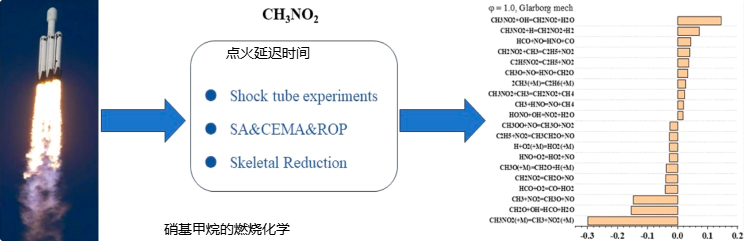
Zhang Y, Zhao Z, Ma R, et al. Experimental and Modeling Study on the Ignition Kinetics of Nitromethane behind Reflected Shock Waves[J]. ACS omega, 2023, 8(42): 39749-39758.
Key Content: The ignition delay time of nitromethane at high fuel concentrations was experimentally and kinetically analyzed. Sensitivity analysis, chemical explosion mode analysis, and reaction path analysis were combined to reveal the fundamental ignition kinetics of NM. A skeletal mechanism for NM ignition and flame speed prediction was developed using directed relation graph-based methods, demonstrating high accuracy.
Future Prospects
Nitromethane has significant potential as a green rocket fuel, but its widespread adoption in the aerospace industry will require further research and development. Future studies will focus on improving combustion efficiency, optimizing ignition methods, and developing ignition systems tailored for satellite and spacecraft propulsion. The resurgence of nitromethane is not merely a supplement to traditional rocket fuels—it marks the beginning of a "renaissance" in propulsion technology!
Next Page
Next Page
Related News


